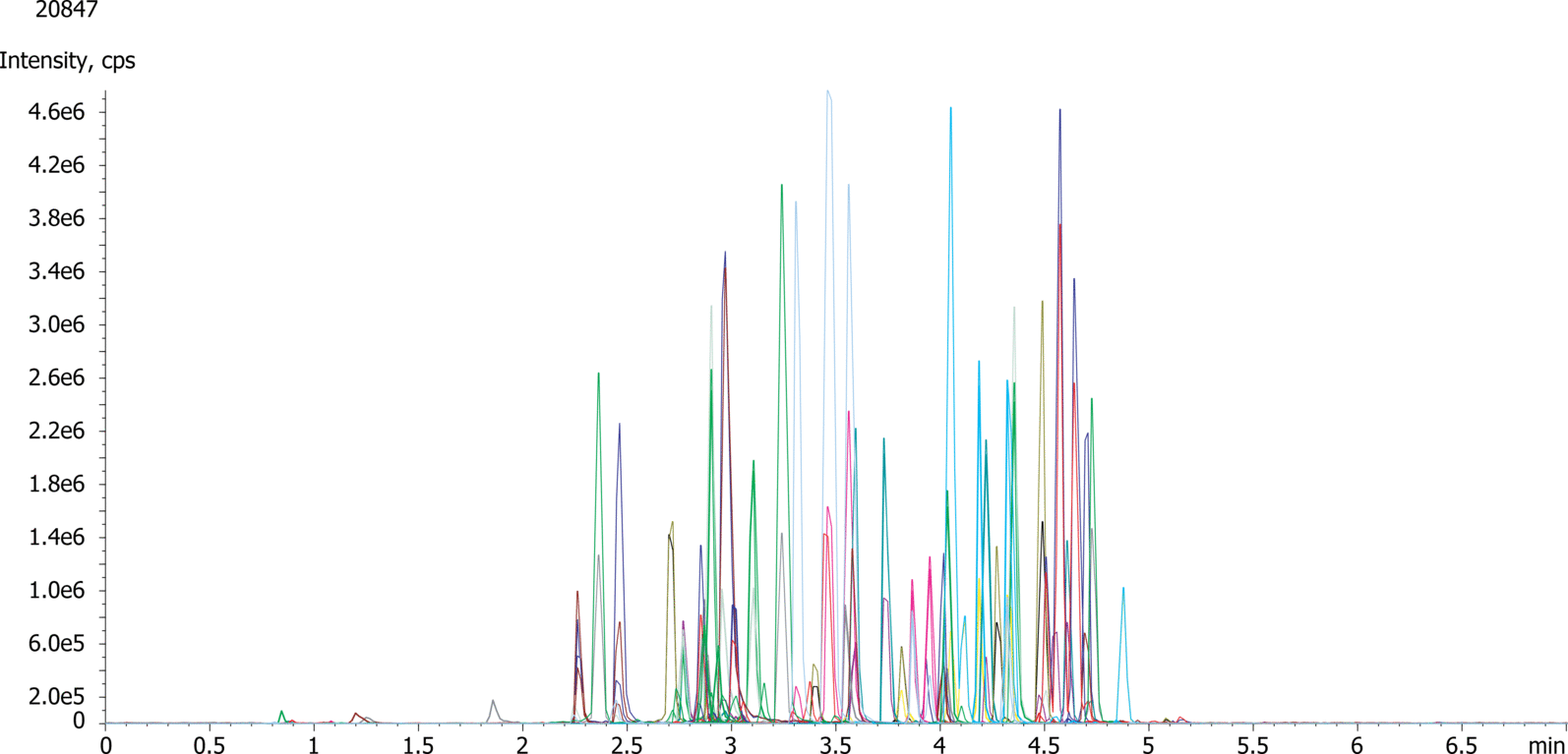
| 1Morphine |
|---|
| 2Morphine-d6 |
| 3Hydromorphone |
| 4Hydromorphone-d6 |
| 5Naloxone |
| 6Codeine |
| 7Oxymorphone |
| 8Naltrexone |
| 9Amphetamine |
| 10Amphetamine-d5 |
| 116-MAM |
| 12Oxycodone |
| 13Hydrocodone |
| 14Hydrocodone-d6 |
| 15Methamphetamine |
| 16MDA |
| 17MDMA |
| 18MDEA |
| 19Norfentanyl |
| 20Tramadol |
| 21Benzoylecgonine |
| 22Benzoylecgonine-d3 |
| 23Meperidine |
| 24Meperidine-d4 |
| 25Normeperidine |
| 26Normeperidine-d4 |
| 27Meprobamate |
| 28Norbuprenorphine |
| 29PCP |
| 30PCP-D5 |
| 31Fentanyl |
| 32Fentanyl-d5 |
| 33EDDP |
| 34Flurazepam |
| 35Norpropoxyphene |
| 36Norpropoxyphene-d5 |
| 37Sufentanil |
| 38Buprenorphine |
| 39Propoxyphene |
| 40Carisoprodol |
| 41Methadone |
| 42Methadone-d3 |
| 43Midazolam |
| 44Lorazepam |
| 45Clonazepam |
| 46Hydroxyalprazolam |
| 47Oxazepam |
| 48Flunitrazepam |
| 49Alprazolam |
| 50Temazepam |
| 51Nordiazepam |
| 52Nordiazepam-d5 |
| 53Diazepam |
| 546-MAM-d3 |
| 55Methamphetamine-d5 |
Compound Name Morphine |
CID 5288826 |
Molecular Formula C17H19NO3 |
Molecular Weight 285 |
No. Hydrogen Bond Acceptors 4 |
No. Hydrogen Bond Donors 2 |