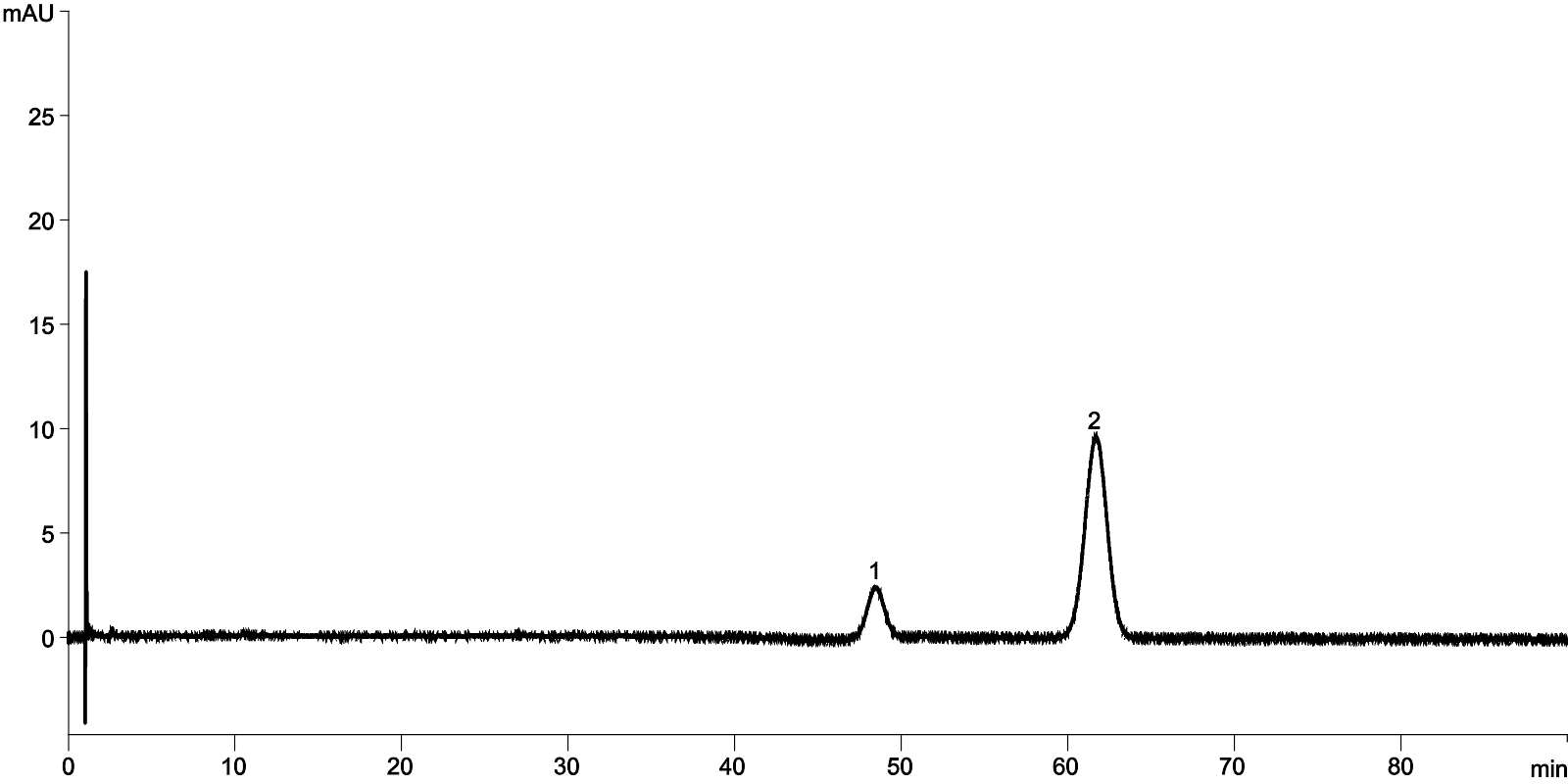
| 1Ritonavir |
|---|
| 2Lopinavir |
Applications
Lopinavir & Ritonavir Tablets USP (COVID-19) Luna 5 µm C8(2) 150 x 4.6 mm
25914
Separation Mode: Reversed Phase
Reversed Phase
USP
C8(2)
HPLC
Anti-HIV Agents
Pharmaceutical/Biopharmaceutical
Therapeutic / Clinical
Therapeutic / Clinical
Analytes
Details
Official Method: USP
LC Conditions (App ID: 25914)
Column: |
Brand Name: Luna |
Part No: |
Phase Name: C8(2) |
System: Agilent Technologies 1260 Infinity Hybrid SFC/UHPLC Agilent Technologies 1260 Infinity Hybrid SFC/UHPLC |
Sample Note: Buffer = 4.1 g Potassium Phosphate monobasic in 1 L of Water
Solution A = 50:50 ACN:Buffer
Samples were dissolved and diluted in Solution A |
Similar Applications
search
No Data