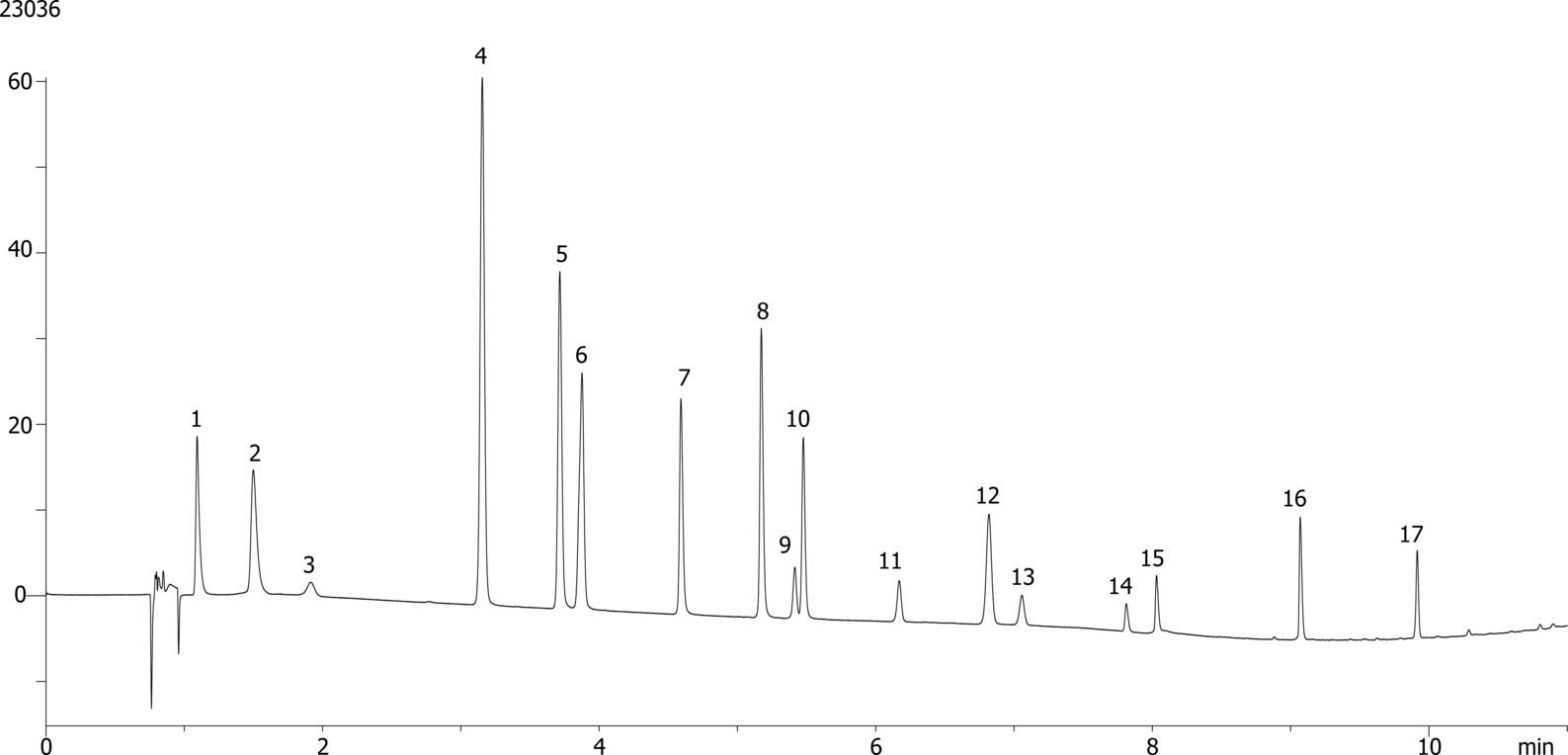
| 14-Nitrophenol |
|---|
| 2Acetaminophen |
| 3Acetylsalicylic acid |
| 4Brompheniramine |
| 5Chlorpheniramine |
| 6Clemastine |
| 7Dextromethorphan |
| 8Diphenhydramine |
| 9Doxylamine |
| 10Fumaric acid |
| 11Guaifenesin |
| 12Ibuprofen |
| 13Impurity |
| 14Maleic acid |
| 15Pheniramine |
| 16Phenylephrine |
| 17Pyrilamine |
Applications
Cold medicine compounds on Kinetex 2.6 µm EVO C18
23036
Separation Mode: Reversed Phase
Reversed Phase
EVO C18
HPLC
Antipruritics,Antiparkinson Agents,Anti-Inflammatory Agents, Non-Steroidal,Antiemetics,Anti-Allergic Agents,Anesthetics, Local,Vasoconstrictor Agents,Sympathomimetics,Platelet Aggregation Inhibitors,Analgesics, Opioid
Pharmaceutical/Biopharmaceutical
Pharmaceutical
Therapeutic / Clinical
Analytes
Details
LC Conditions (App ID: 23036)
Column: |
Brand Name: Kinetex |
Part No: |
Phase Name: EVO C18 |
Sample Note: Forced degradation protocol:
Reconstitute 1 mg of cefaclor neat standard with 1.0 mL of 1 M HCl. Heat the solution at 60 ºC for 6 hours then neutralize with 1.0 mL of 1 M NaOH. Refrigerate until ready to inject. |
Similar Applications
No Data