Blog
Best and Worst Organic Solvents to Use in LC
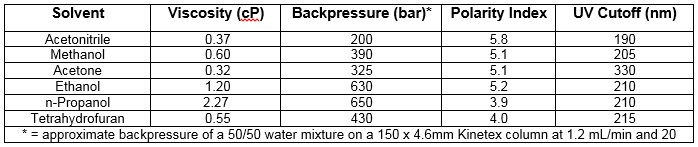
There are several critical characteristics to take into consideration when choosing the appropriate organic solvent to use in liquid chromatography. For example, high viscosity solvents may produce backpressures that are too high for the HPLC system used.
Posted on Jul 14, 2016
There are several critical characteristics to take into consideration when choosing the appropriate organic solvent to use in liquid chromatography (see table at bottom). Of them, viscosity is one of the most crucial, as high viscosity solvents may produce backpressures that are too high for the HPLC system used.
Other important solvent characteristics include UV cutoff and polarity index: high UV cutoff will result in poor sensitivity with UV/Vis detection, while solvents with low polarity indices generally result in faster elution of organic compounds and are commonly used for column cleaning.
 Iso- and n-propanol have relatively strong elution strength and are most commonly used in column cleaning at low flow rates as they also result in high backpressures. These make good solubilizers and are often preferred with intact proteins.
Tetrahydrofuran has similar elution strength to n-propanol, but is less commonly used as it is much more expensive. However, it can be used as a cleaning or regeneration solvent for fatty or highly pigmented samples.
Iso- and n-propanol have relatively strong elution strength and are most commonly used in column cleaning at low flow rates as they also result in high backpressures. These make good solubilizers and are often preferred with intact proteins.
Tetrahydrofuran has similar elution strength to n-propanol, but is less commonly used as it is much more expensive. However, it can be used as a cleaning or regeneration solvent for fatty or highly pigmented samples.
Related resources: • Solvent and Temperature Considerations • Understanding the Revisions to USP Monograph <467>: Residual Solvents • HPLC Troubleshooting Guide • HPLC Column Care Guide
BEST: Acetonitrile, Methanol, Acetone
Acetonitrile is arguably the best organic solvent as it results in the lowest system backpressure in water mixtures and also has a very low UV cutoff for better UV/Vis detection sensitivity. Methanol is another popular organic solvent as it is comparable in elutropic strength to acetonitrile, has a relatively low UV absorbance, and is significantly less expensive than acetonitrile. The main drawback of methanol, especially when used with small particle size HPLC columns, is that its use can result in backpressures that exceed many HPLC system limits.Rock your LC lab. Get free earbuds. >>>
Despite its high UV absorbance, acetone can be utilized successfully if analytes absorb at higher UV wavelengths, or if other detector types such as mass spec or ELSD are used.WORST: Ethanol, Iso-/n-propanol, Tetrahydrofuran
Ethanol is generally not recommended as it results in very high backpressures in water mixtures. Sometimes, however, ethanol is preferred in finished product preparative methods to avoid residual solvents. Iso- and n-propanol have relatively strong elution strength and are most commonly used in column cleaning at low flow rates as they also result in high backpressures. These make good solubilizers and are often preferred with intact proteins.
Tetrahydrofuran has similar elution strength to n-propanol, but is less commonly used as it is much more expensive. However, it can be used as a cleaning or regeneration solvent for fatty or highly pigmented samples.
Iso- and n-propanol have relatively strong elution strength and are most commonly used in column cleaning at low flow rates as they also result in high backpressures. These make good solubilizers and are often preferred with intact proteins.
Tetrahydrofuran has similar elution strength to n-propanol, but is less commonly used as it is much more expensive. However, it can be used as a cleaning or regeneration solvent for fatty or highly pigmented samples.

Related resources: • Solvent and Temperature Considerations • Understanding the Revisions to USP Monograph <467>: Residual Solvents • HPLC Troubleshooting Guide • HPLC Column Care Guide














