Degassing Techniques in HPLC: Inline vs Offline
October 31, 2025
Author: Our Phenomenex Team
Air bubbles in an HPLC system can disrupt pumping reliability, cause baseline noise, and result in poor result reproducibility and valve function. To prevent these, proper mobile phase degassing is essential. Degassing in HPLC not only ensures stable pumping but also improves sensitivity and accuracy by preventing dissolved oxygen from forming bubbles that interfere with detectors such as UV-VIS, fluorescence, refractive index, or electrochemical. Effective HPLC solvent degassing is therefore a critical step for stable, accurate, and reproducible chromatography.
What is HPLC Solvent Degassing and Why is it Necessary?
When solvents such as water, buffers, acetonitrile, and methanol contact the atmosphere, nitrogen and oxygen dissolve into them. When mixed, their combined air content can exceed the solubility of the mixture, creating a supersaturated condition that leads to outgassing and bubble formation. These bubbles disrupt HPLC performance by causing unstable baselines, bad peak shape, pump cavitation, retention time shifting, and loss of sensitivity.
In pumps, air can stop flowing or cause erratic retention times, while in optical detectors, bubbles scatter light and generate noise or false signals. Although it is not necessary to remove all dissolved air, degassing in HPLC lowers gas levels below supersaturation and prevents these issues. Low-pressure mixing systems are especially prone to bubble formation, whereas high-pressure mixing reduces outgassing because solvents blend under elevated pressure.
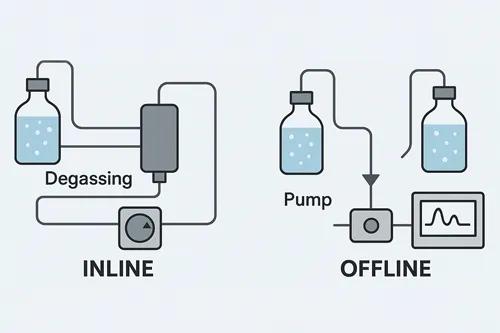
Types of Degassing Techniques in HPLC
Degassing the mobile phase was once a major concern in liquid chromatography with outgassing problems being among the most common issues during the first decades of its use. The introduction of inline degassers in HPLC in the past 15–20 years has significantly reduced the occurrence of these problems, to the point that many users today have little experience with them. However, while inline degassing prevents most solvent outgassing issues, it does not eliminate all problems caused by dissolved air.
Inline Degassing
Inline degassing works by passing the mobile phase through a gas-permeable membrane within a vacuum chamber, allowing dissolved gases to escape while retaining the liquid. Different techniques include:
- Vacuum degasser: Inline vacuum degassers, now standard in most modern HPLC systems, have greatly reduced bubble-related pump issues by efficiently removing dissolved gases. Solvents pass through porous tubing within a vacuum chamber, where gases diffuse out through micropores to waste, while the degassed liquid continues flowing onto the pump.
- Membrane degasser: Over the years, various polymer materials have been tried with varying success for degassers. Modern degassers use a thin, highly gas-permeable membrane, making the degassing process very efficient.
Advantages:
Inline degassers are highly reliable and effective at reducing dissolved gases in the mobile phase to acceptable levels and are now standard in most HPLC systems. It is usually automatic and trouble-free. They require little to no maintenance, making them convenient and reliable for routine use.
Limitations:
Despite their effectiveness, inline degassers do not remove all dissolved air from the mobile phase. The residual air can still cause problems in certain sensitive applications. Membrane replacement, vacuum pump failure, or damaged tubing can also cause issues, so proper maintenance is essential to avoid costly repairs. Additionally, microbial growth in the buffer can block membrane pores and contaminate the entire HPLC system. To prevent microbial growth, contamination, or blockages, it is best not to store the degasser with water or buffer in the tubing and to flush with 30% phosphoric acid followed by water if contamination occurs.
Offline Degassing
Offline degassing involves removing dissolved air from the mobile phase before it enters the HPLC system. The techniques include:
- Helium sparging: This method requires a solvent inlet filter to prevent particulates and improve gas dispersion, but the degassed solvent can reabsorb air once in the system. To reduce bubble formation, slight back pressure should be maintained, often using a backpressure regulator after the detector. Helium purging can remove up to 80% of dissolved air, requiring equal helium volume for organic–aqueous phases. However, the flow rate should be reduced over time to avoid losing volatile components.
- Vacuum filtration: Vacuum degassing removes over 60% of dissolved air from the mobile phase and is performed through batch-wise treatment. It can also be done during filtration, before the chromatographic process with a 0.45 or 0.22 μm membrane filter, though this method is less effective.
- Sonication: Ultrasonic baths alone are not effective for degassing, as they remove only about 20-30% of dissolved gases, while at least 50% removal is needed. However, when combined with helium sparging or vacuum degassing, sonication becomes a useful complementary method. Since ultrasonic baths are common in laboratories, they are best applied alongside other degassing techniques.
Advantages:
An offline degasser offers the advantage of using a 2 µm solvent inlet filter to prevent particulates from entering the mobile phase while ensuring efficient gas dispersion. It is also cost-effective and flexible, as it relies on standard laboratory equipment and can handle large solvent volumes in a batch.
Limitations:
A major drawback of offline helium sparging is that the mobile phase can gradually reabsorb air once placed in the HPLC system, leading to signal loss and changing sensitivity. Additionally, helium sparging can be expensive as it requires specialized gas cylinders.
Inline vs Offline Degassing: Side-by-Side Comparison
| Aspect | Offline Degassing | Inline Degassing |
|---|---|---|
| Timing | It is usually done before the mobile phase enters the HPLC run. | It is a continuous process that occurs during an HPLC run. |
| Degassing Efficiency | The efficiency can be variable. For instance, vacuum/sonication removes 60–70% of dissolved air, sonication alone removes 20–30% of air and helium sparging removes 80% of air. | It does not remove 100% of dissolved gas, but it removes most of it. However, it prevents bubble formation. |
| Risk of Re-dissolving | The risk is high as solvents can reabsorb air when exposed to the atmosphere. | The risk is low as the continuous vacuum keeps new gas from dissolving. |
| Maintenance | Clean glassware and filters as needed. No special parts. | Membrane degassers are durable. Cleaning is needed if it is clogged. Hence, low maintenance. |
| Cost | Low initial investment and requires only basic lab equipment. Helium sparging can be expensive as it requires specialized gas cylinders. | Higher initial cost (dedicated degasser unit and vacuum pump). |
| Ease of Use | Manual setup each time which is time-consuming. | Automated after installation. |
| Impact on Solvent Composition | Some loss of volatiles is possible such as evaporation during sparging. | Solvent composition remains unchanged. |
| Effect on Chromatographic Stability | Improves initial stability but may allow bubble re-formation later. | Very stable baselines and retention. Bubble-related problems are virtually eliminated. |
When to Choose Offline vs Inline Degassing
The choice between an offline and inline HPLC degasser depends on the situation, including factors like routine use, troubleshooting needs, and method sensitivity.
- Offline degassing is suitable for occasional use, limited equipment setups, or when routine inline degassing is not available. While inline degassing is preferred for routine analyses requiring consistent performance and high sensitivity.
- Inline degassers have a higher initial cost but save labor. An offline degasser uses low-cost labware and is cheap to set up, though helium or other gases add to the consumable cost.
- If mobile phases contain volatile organics or labile salts, avoid aggressive offline sparging/vacuum (which can evaporate components). Inline vacuum degassing is gentler on the solvent composition.
- Oxygen in the mobile phase is particularly problematic for sensitive methods, such as low-wavelength UV or reductive electrochemical detection. While techniques like helium sparging and inline degassing effectively remove oxygen, remember that offline-sparged solvents are susceptible to reabsorbing air over time, which can lead to signal loss and subtle changes in sensitivity.
Troubleshooting Degassing Issues in HPLC
Cycling of output (pressure or baseline)
Cycling patterns in pressure or the chromatogram baseline often indicate poor compressibility at the pump heads. This may result from insufficient degassing efficiency, where adding a second degassing chamber can help. It can also cause air to enter the system due to improperly connected tubing to or from the HPLC solvent degasser. Also, improper connections of the tubing on the HPLC solvent degasser can also allow air to enter the system, causing fluctuations in pressure or baseline.Baseline noise
Noise on the baseline of a chromatogram may indicate problems with the gas flow system. High gas flow rates can cause short-term noise, while baseline drift (long-term fluctuations) may be due to contaminants being flushed out from a partially clogged gas line. Additionally, an unstable gas flow output can also contribute to baseline noise.Leakages
Leaks often occur due to incompatibility between solvents and degasser materials. For instance, tetrahydrofuran (THF) can dry and crack degasser membranes. Additionally, improperly seated tubing connections in or out of the degasser can allow leaks to develop.Blockages
Blockages create pressure ripples as the pump struggles to draw solvent, sometimes causing cavitation. They may result from algae growth, crystallization of buffer salts, or clogged inlet filters. Troubleshooting involves disconnecting outlet tubing to check for continuous solvent flow, while prevention requires filtering solvents, using clean solutions, and flushing lines regularly, especially after system shutdowns.General considerations
Degasser issues are often linked with the mobile phase quality. Using poorly prepared or contaminated solvents can lead to persistent problems. Maintaining clean, filtered, and fresh solvents helps ensure smooth operation and minimizes downtime.
FAQs
What is the difference between open and closed system degassing?
Open system degassing, such as helium sparging in an open container, allows the solvent to contact ambient air, which increases the risk of gases re-dissolving. Closed system degassing, like inline vacuum or membrane degassers, isolates the solvent from air while continuously removing dissolved gases. This makes closed systems more efficient and stable for maintaining bubble-free solvents during analysis.
Which degassing method ensures the highest removal of dissolved gases?
Among offline methods, helium sparging is the most effective, removing up to about 80% of dissolved gases. Inline vacuum or membrane degassers provide continuous and highly efficient removal, keeping solvents bubble-free throughout the run. For maximum gas removal, combining helium sparging with inline degassing offers the highest reliability.
Can a combination of degassing methods be used?
Yes, a combination of degassing methods can be used to enhance efficiency. For example, solvents can first be treated with helium sparging or sonication offline, then passed through an inline vacuum degasser. This layered approach minimizes residual gases and ensures greater chromatographic stability.
What special considerations exist for oxygen-sensitive analyses?
For oxygen-sensitive analyses, it is important to minimize dissolved oxygen in the mobile phase. Inline vacuum or membrane degassers combined with helium sparging are preferred for extra protection. Additionally, solvent reservoirs should be sealed or kept under an inert gas blanket to prevent oxygen re-entry.